Fundamentals of fuel cell
Unlike conventional power plants, the fuel cell’s chemical energy is converted directly into electrical energy. The detour of the conversion of chemical energy into heat, ie mechanical energy (turbine) and electrical energy (generator) is avoided.
The fuel cell is the future of direct energy conversion. An inexhaustible innovation potential and a number of advantages indicate that this technology will spread quickly and comprehensively. Not only specific types of fuel cells, but also intelligent test systems for the most diverse applications are offered by us.
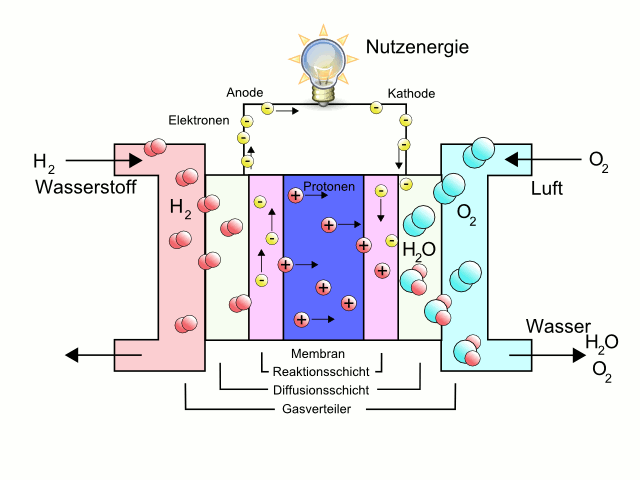 In a fuel cell, using hydrogen (H2) and oxygen (O2), chemical is converted into thermal and electrical energy.
In a fuel cell, using hydrogen (H2) and oxygen (O2), chemical is converted into thermal and electrical energy.
The hydrogen is split by the catalyst into protons and electrons.
Here then the electrical work is done at the consumer.

The total voltage of a fuel cell is thus 1.23 V. Practically, however, this value is not reached because the fuel cell also has internal losses.
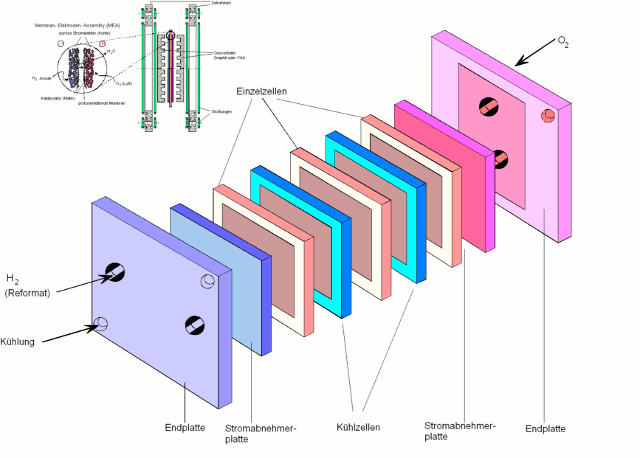
Structure of a fuel cell stack
To achieve higher voltage, multiple fuel cells are mounted in stacks in series.
A stack of fuel cells consists of end plate, pantograph plate, the respective fuel cells, which alternate with cooling cells and finally again a pantograph plate and end plate.

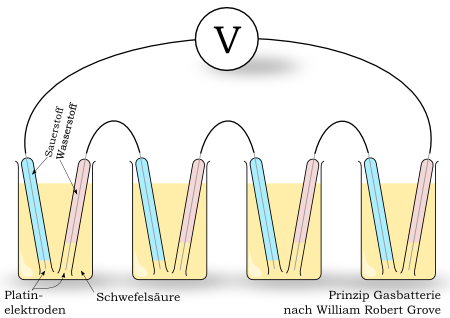 Im Jahr 1839 beschrieb der Engländer Sir William Robert Grove die kalte Verbrennung – das Grundprinzip der Brennstoffzelle. Aufgrund fehlender Technologien und dem Bau des ersten praktisch verwendbaren Generators durch Werner von Siemens geriet die Brennstoffzelle jedoch wieder in Vergessenheit.
Im Jahr 1839 beschrieb der Engländer Sir William Robert Grove die kalte Verbrennung – das Grundprinzip der Brennstoffzelle. Aufgrund fehlender Technologien und dem Bau des ersten praktisch verwendbaren Generators durch Werner von Siemens geriet die Brennstoffzelle jedoch wieder in Vergessenheit.